Protean MAPS™ Improves the Delivery of Precision Health Information to Community Oncology Practices
Originally presented at ASCO – May 25th 2023
Anthony Martin Magliocco, Roy Khalife, Catherine Wilson, Tara M Love, Rama Balaraman
Protean BioDiagnostics, Orlando, FL; Roche Information Solutions, Sunnyvale, CA; Ocala Onc, Ocala, FL
Abstract:
DelPHI: Improves the Delivery of Precision Health Information
A novel program to accelerate access to precision oncology in the community oncology setting.
Background
It has been well recognized that the rapid advances in precision oncology have not fully been translated into improved access for community cancer programs in the USA, where as much as 95% of oncological assessment and treatment occurs.
To address this growing gap, Protean BioDiagnostics and Roche Information Solutions have created a novel project to improve the Delivery of Precision Health Information (DelPHI) to community-based oncology programs using an innovative precision oncology testing system (MAPS™) integrated with a digital decision support and telemedicine framework (navify).
The project seeks to bridge the clinical and administrative gaps to help bring precision oncology to the community.
Methods
In an initial implementation of DelPHI, 28 cases were analyzed from a community oncology clinic. Analyses included immunohistochemistry (IHC) testing, rapid molecular testing, and a 523-gene DNA, 55-gene RNA comprehensive cancer panel. These cases were grouped together and queried for IA actionability alterations, potentially actionable alterations, potentially germline, pathogenic variants, time to each step, diagnosis changes, and noted if there were any inadequate case materials.
Results
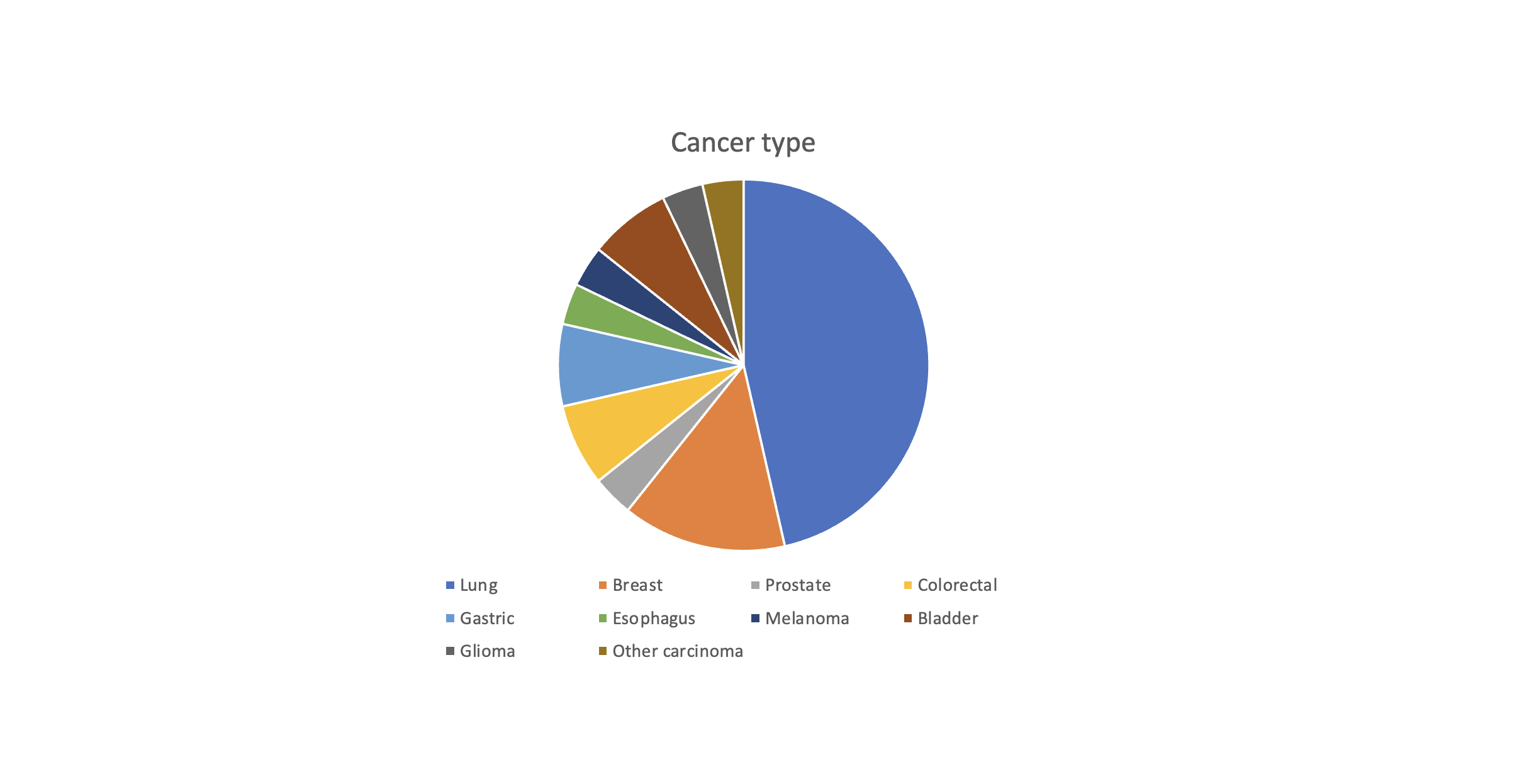
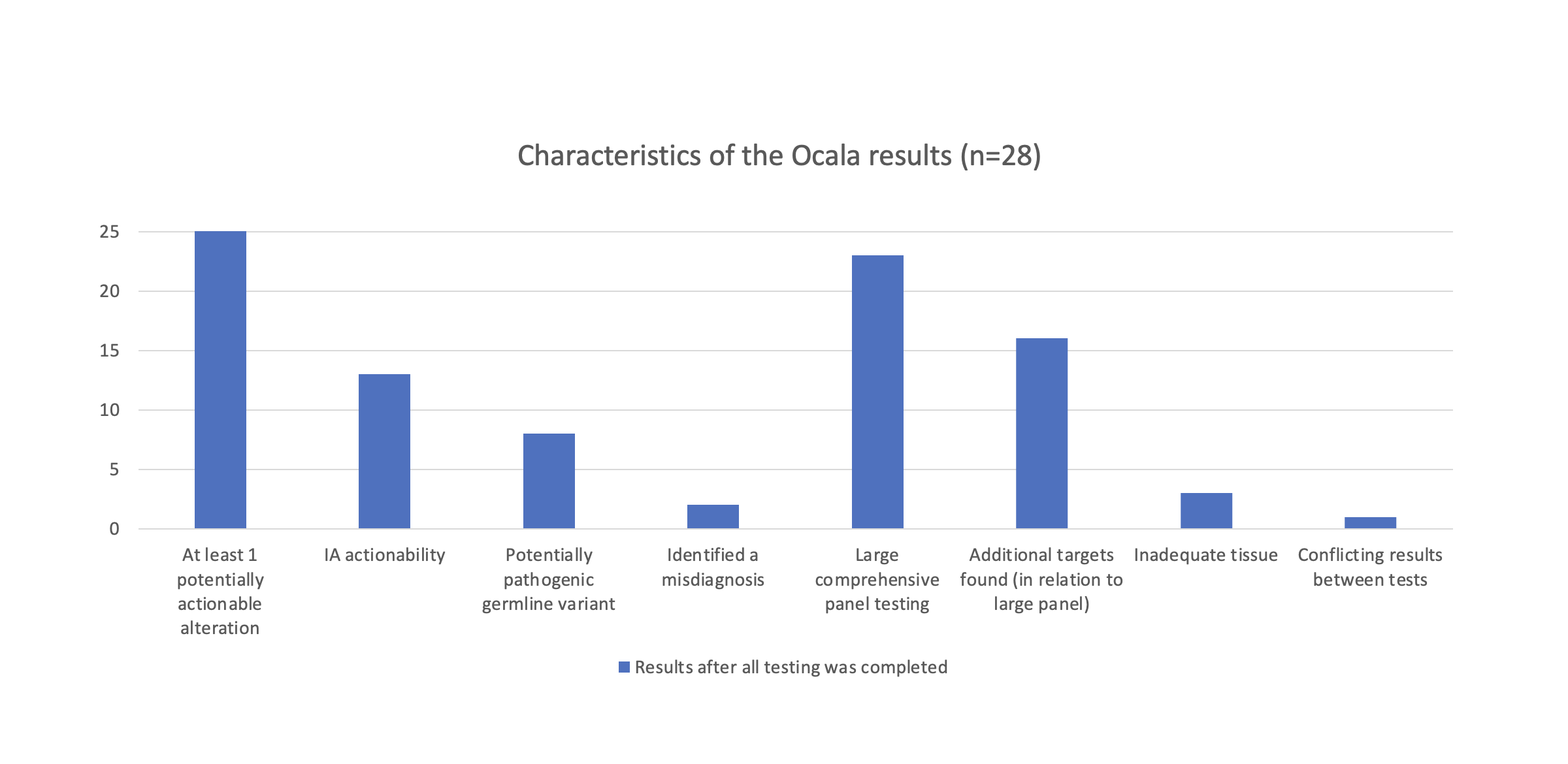
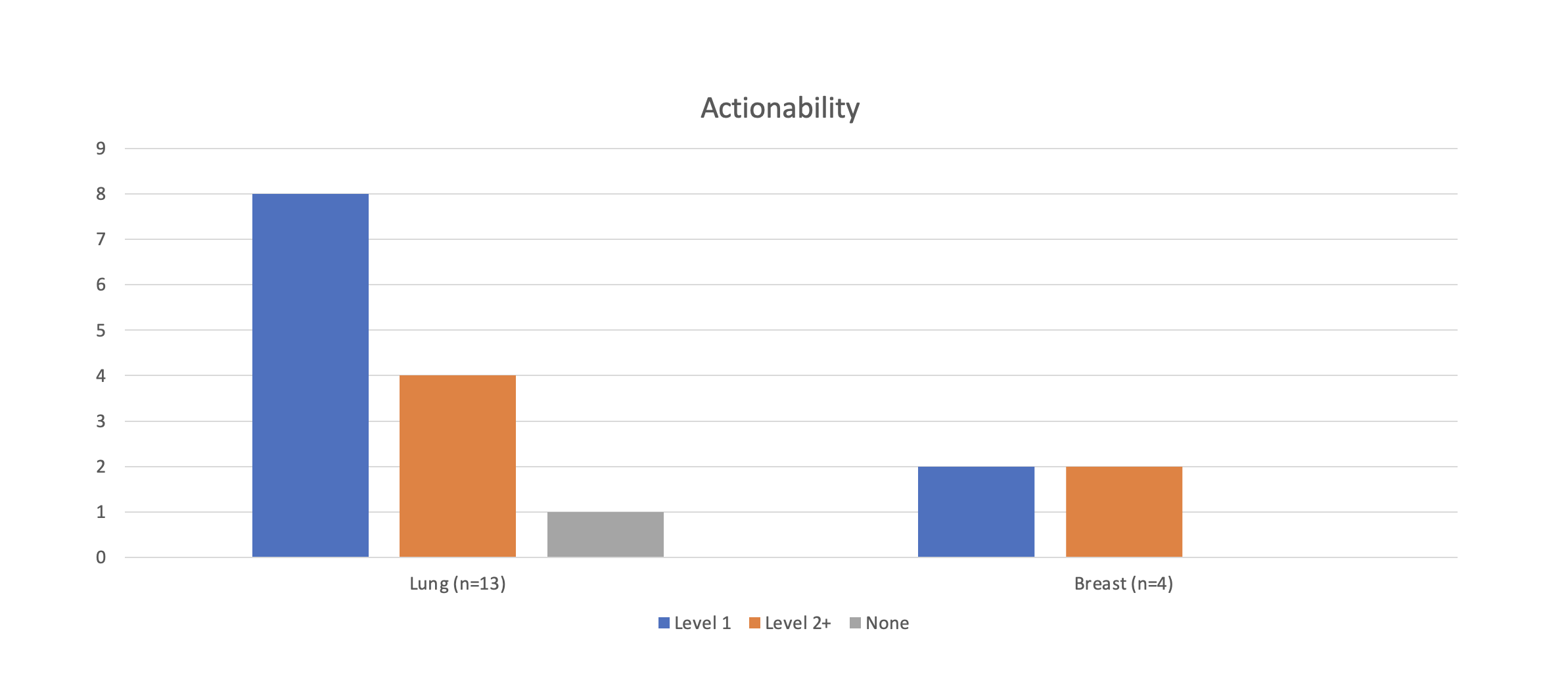
Based on the 28 patients that were run (13 lung, 4 breast, 1 prostate, 2 colorectal, 2 gastric, 1 esophagus, 1 melanoma, 2 bladder, 1 glioma, 1 poorly differentiated carcinoma), 50 total actionable alterations were detected. 90% of the patients had at least one potentially actionable alteration, 46% of patients had a IA actionability alteration, and 29% of patients had a potentially pathogenic germline variant that may warrant further testing. We identified 2/28 patients with incorrect diagnoses that were corrected after testing. 23/28 patients underwent testing via the large, comprehensive panel, and 16 of these 23 had additional targets. 3 individuals had inadequate tissue to analyze, and 1 patient had an ALK expression imbalance that was not seen in other tests, which may be due to heterogeneity of the sample; both of these issues may benefit from liquid biopsies.
Conclusions
Precision medicine can be delivered to community cancer centers using a combined integrated molecular testing system coupled with a digital knowledge support framework leading to improved speed of patient testing and uncovering of actionable biomarkers. Numerous challenges were identified, including delays in accessing tissue and limited tissue availability. These challenges could be overcome with alternative testing approaches, such as liquid biopsy and education to improve strategies for tissue collection and management.
Research Sponsor: Roche Diagnostics Corporation.
For further information, please contact:
Anthony Magliocco MD
CEO & President of Protean BioDiagnostics
Email: info@proteanbiodx.com
Phone: 1 (813) 817 2042
About Protean BioDiagnostics
Protean is an innovative diagnostic company developing new approaches to improving precision medicine including inventing digital pathology tools to improve cancer screening and detection.
Protean MAPS™ is the only unique precision medicine system that provides easy access to advanced comprehensive laboratory diagnostic tests and easy to understand reports along with telemedicine support. Protean’s mission is to accelerate and bring optimized precision care to all cancer patients regardless of where they live.

